DNA & Nucleotide Modification
DNA crosslinks are a highly cytotoxic form of DNA damage. DNA damage repair enzymes play a vital role in the repair of such DNA lesions. DNA crosslinking is exploited by some anti-cancer chemotherapeutics and therefore, inhibition of DNA repair enzymes could improve the success of such drugs.
The 5’-3’ exonuclease SNM1A is a key enzyme involved in the repair of interstrand crosslinks, a cytotoxic form of DNA damage. It digests past DNA lesions with 5’-3’ directionality by hydrolysing the phosphodiester backbone. Due its implication in resistance to anti-cancer therapeutics, SNM1A is a potential target for anti-cancer chemotherapy and it is, therefore, important to fully understand its mechanism of action.
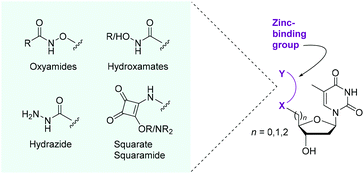
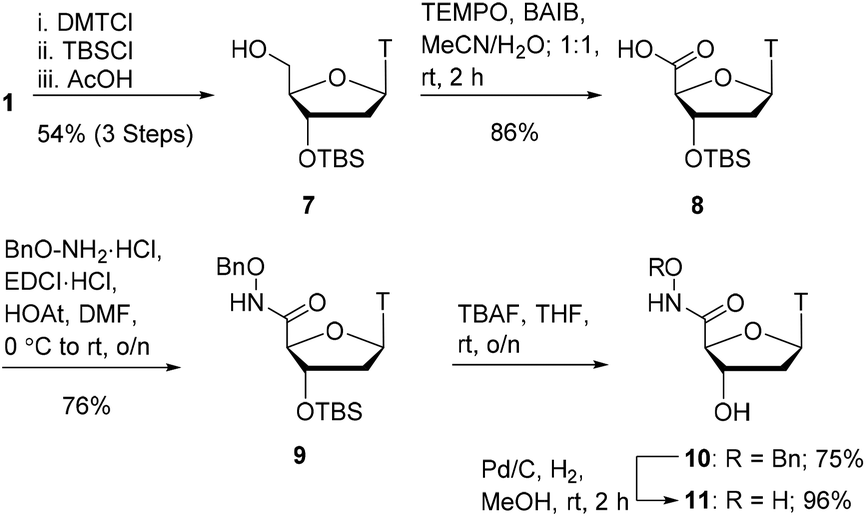
Research in the group focuses on the synthesis of inhibitors and activity-based probes for poorly characterised nucleases such as SNM1A. These inhibitors are based on DNA, the natural substrate of SNM1A, and are synthesised through modification of nucleosides and nucleotides. Activity of the enzyme depends on metal ions in the active site, and hence, our research approach focuses on the incorporation of metal binding groups onto nucleosides and nucleotides. To date, we have successfully generated effective inhibitors for SNM1A based both on mononucleosides and oligonucleotides bearing various metal binding groups. Effective inhibitors have the potential to be developed into activity-based probes through incorporation of probe elements such as photocrosslinking groups and affinity handles.

Relevant Publications
Berney, M; Fay, EM; Doherty, W; Deering, JJ; Dürr, EM; Ferguson, S; McGouran JF. Zinc-Binding Oligonucleotide Backbone Modifications forTargeting a DNA-Processing Metalloenzyme ChemBioChem, 2024, doi: 10.1002/cbic.202400528
Arbour, C. A.; Fay, E. M.; McGouran, J.F,: Imperiali, B. Deploying solid-phase synthesis to access thymine-containing nucleoside analogs that inhibit DNA repair nuclease SNM1A Organic & Biomolecular Chemistry, 2023, 21, 5873-5879
Fay, E. M.; McGouran, J. F. Exploring the binding pockets of the DNA damage repair enzyme SNM1A through nucleobase modification Results in Chemistry, 2023, 5, 100939
Fay, EM; Newton, A; Berney, M; El-Sagheer, A; Brown, T; McGouran, JF. Two-Step Validation Approach for Tools to Study the DNA Repair Enzyme SNM1A, ChemBioChem, 2021, e202200756
Berney, M; Manoj, MT; Fay, EM; McGouran, JF., 5'-Phosphorylation increases the efficacy of nucleoside inhibitors of the DNA repair enzyme SNM1A, ChemMedChem, 2021, e202100603
Dürr, E. M. & McGouran, J.F. Probing the binding requirements of modified nucleosides with the DNA nuclease SNM1A, Molecules, 2021
Doherty, W; Dürr, E. M.; Baddock, H; Lee, S. Y.; McHugh, P. J.; Brown. T; Senge, M.O.; Scanlan, E.M.; McGouran, J. F.; A Hydroxamic-acid-containing nucleoside inhibits DNA repair nuclease SNM1A, Organic & Biomolecular Chemistry, 2019
Thomas A, Brolih S, McGouran J, El-Sagheer A, Ptchelkine D, Jones M, McDonald N, McHugh P, Brown T, Optimised oligonucleotide substrates to assay XPF ERCC1 nuclease activity for the discovery of DNA repair inhibitors, Chemical Communications, 2019
Dürr, E. M.; Doherty, W; Lee, S. Y.; El‐Sagheer, A. H.; Shivalingam, A; McHugh, P. J; Brown, T; McGouran, J. F; Squaramide‐Based 5’‐Phosphate Replacements Bind to the DNA Repair Exonuclease SNM1A, ChemistrySelect, 2018
Berney, M; McGouran, J. F., Methods for detection of cytosine and thymine modifications in DNA, Nature Reviews Chemistry, 2018
Abdullah U.B., McGouran J.F., Brolih S, Ptchelkine D, El‐Sagheer A.H., Brown T, McHugh P.J., RPA activates the XPF-ERCC1 endonuclease to initiate processing of DNA interstrand crosslinks, The EMBO journal, 2017
Lercher L, McGouran J.F., Kessler B.M., Schofield C.J., Davis B.G., DNA modification under mild conditions by Suzuki-Miyaura cross-coupling for the generation of functional probes., Angewandte Chemie (International ed. in English), 52, (40), 2013, 10553-8